InMotion® Robotic Therapy
InMotion® Robotic Therapy improves upper-extremity motor retraining for patients following neurological conditions and injuries.
- The InMotion® Arm, and Arm/Hand enhance the therapist’s ability to drive repetition and neuroplasticity
- Helps to restore motor function and improve outcomes.
What do Clinicians and Patients Say about InMotion® Interactive Therapy?
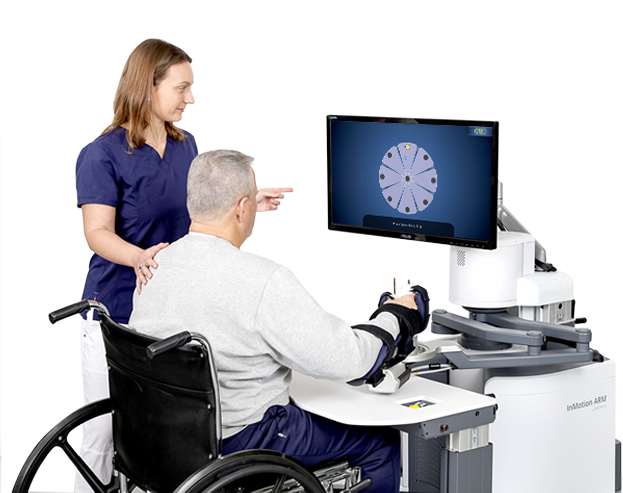
Use of the InMotion® ARM for Stroke and SCI rehabilitation at The Tallahassee Memorial Rehabilitation Center
Extensive Evidence Base: 25+ years of Research and more than 150 Independent Peer-reviewed Publications
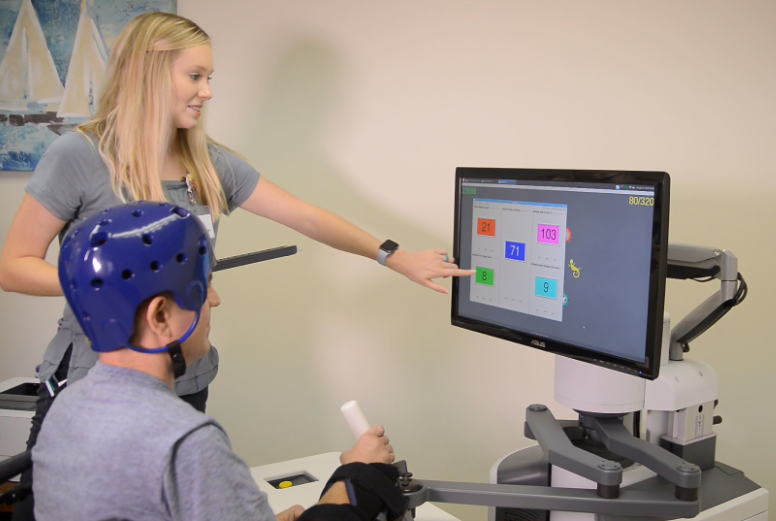
Improved Outcomes Across a Wide Range of Patient Populations:
- Adult and Pediatric patients (4 years and older)
- Acute, subacute and chronic phases of recovery
- Severe, moderate and mild levels of impairment
BIONIK’s modular approach to neurorehabilitation is optimizes the use of robotics and is consistent with the latest neuro-scientific research. InMotion® Interactive Therapy serves as an effective adjunct to existing physical and occupational therapy approaches.
Chronic Stroke Treatment: Clinically Significant Results
Mr. Rogers. TMH Patient undergoing treatment for Stroke
In a multi-center, randomized controlled trial involving 127 chronic stroke patients with moderate to severe upper-limb impairment, InMotion therapy demonstrated significant findings:
- Improvement in arm movement, function and quality of life.
- Evidence of potential long-term benefits.
- Challenge the widely held clinical belief that gains in motor function are not possible for long term stroke survivors.” 1

Double the Impairment Reduction!
In a controlled clinical study involving 56 stroke inpatients, the motor skills of the robot-treated group improved significantly more than the control group. Analysis showed that interactive robotic therapy significantly reduced motor impairment of the treated limbs, doubling the impairment reduction. 2
- Lo. A.C., et.al “Robot-Assisted Therapy for Long-Term Upper Limb Impairment after Stroke,” New England Journal of Medicine, 362:1772, May 13, 2010
- Volpe, B.T., Krebs, H.I., Hogan, N., Edelstein, O.L., Diels, C and Aisen, M., A Novel Approach to Stroke Rehabilitation Robot-Assisted Sensori-motor Stimulation, Neurology, 54 (2000) 1938-44
Advanced Technology: Powered by Robotic A.I.

BIONIK robotic products have exceptional capacity for patient assessment and real-time interactive response, which sets them apart from other therapy systems.
- Senses the patient’s movement and responds to a patient’s continually-changing ability
- Robots guide the exercise treatment accordingly
- If the patient is unable to move, the robot gently assists the patient to initiate movement towards the target
- If coordination is a problem, the robot “guides” the movement, allowing the patient to move towards the target and making certain that the patient is practicing the movement the correct way
- As the patient gains movement control, the robot provides less assistance and continually challenges the patient
- Provides quantifiable feedback on progress and performance

